[18F]FES is FDA-approved since 2020, and is now distributed in Israel by S.R.Y Medical Services.
[18F]FES is an analogue of estradiol that binds the estrogen receptor (ER) with high affinity and selectivity1.
It is indicated for detecting ER-positive lesions, as an adjunct to biopsy, in patients with recurrent or metastatic breast cancer2.
Current SNMMI/ EANM Appropriate Use Criteria for ER-Imaging of Breast Cancer Patients using [18F]FES deem the following indications as appropriate3,4:
- Assess ER status in lesions that are difficult to biopsy or when biopsy is nondiagnostic.
- Guide therapy at the initial presentation of metastatic disease or after progression of metastatic disease.
- Detect ER-expressing breast cancer lesions when other imaging tests are equivocal or suggestive.
Emerging indications requiring more evidence3,4:
- Detect ER-expressing lesions in patients with suspected/ known recurrent or metastatic breast cancer.
- Assess ER status, in lieu of biopsy, in lesions that are easily accessible for biopsy.
- Stage invasive lobular breast cancer (ILC) and low-grade ER-expressing invasive ductal cancer.
- Routinely stage ER-expressing extra-axillary nodes and distant metastases.
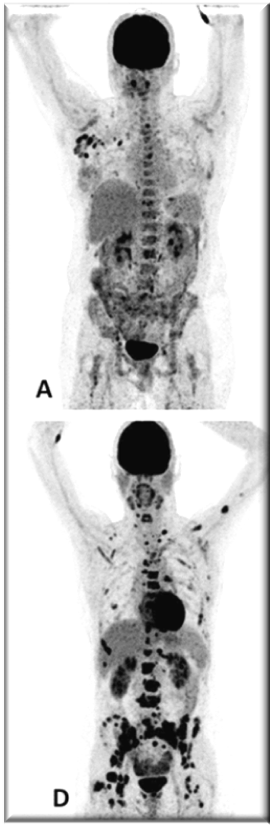
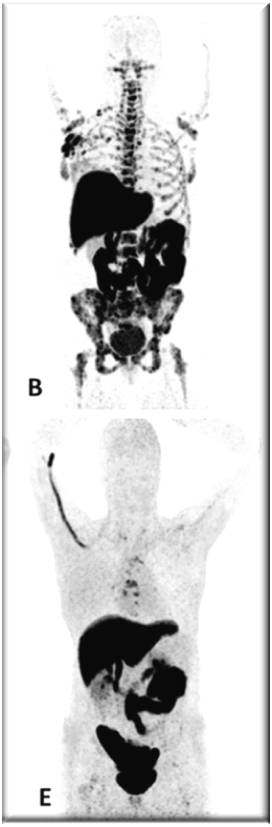
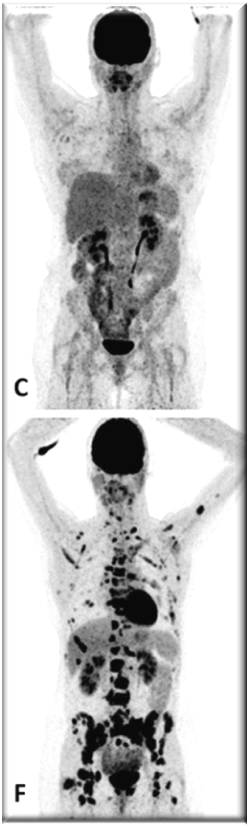
PET images of two patients with metastatic invasive lobular carcinoma responding (A-C) and not responding (D-F) to palbociclib + letrozole
Adapted with permission from Boers, J., et al. European Journal of Cancer. 2020; 126: 11-20
Upper row responder: (A) Baseline [18F]FDG-PET shows pathological uptake in axillary lymph nodes (right side) and in nearly all vertebrae and pelvic bones. (B) Baseline [18F]FES-PET with pathological ER expression in the axial skeleton (vertebrae, pelvic bones, proximal humeri and femora) and in axillar lymph nodes. (C) [18F]FDG-PET after 8 weeks shows almost complete metabolic response. The patient has been on treatment for more than 70 weeks.
Lower row non-responder: (D) Baseline [18F]FDG-PET shows pathological uptake in multiple skeletal lesions. (E) Baseline [18F]FES-PET with only some increased ER expression in thoracic vertebrae. (F) [18F]FDG-PET after 8 weeks shows no metabolic response, with some increase in the pathologic uptake in the multiple skeletal lesions.
- Katzenellenbogen, J. A., et al. Nucl Med Biol. 2021; 92: 24
- https://www.gehealthcare.com/-/jssmedia/GEHC/US/Files/Products/Nuclear-Imaging-Agents/Cerianna/cerianna-pi
- Ulaner, G. A., et al. J Nucl Med. 2023; 64: 351
- Mankoff, D., Balogová, S., et al. J Nucl Med. 2024; 65: 221




